Making the flu vaccine: a race against the clock

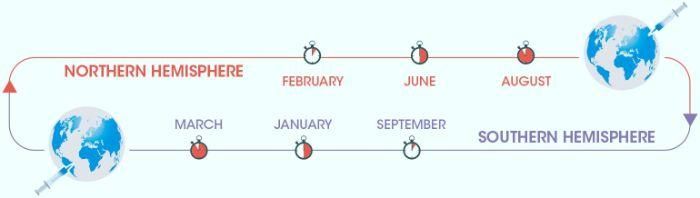
For days, at the sites of Sanofi Pasteur(1) in Val de Reuil in France, Swiftwater in the United States, Shenzhen in China and Ocoyoacac in Mexico, a fleet of trucks delivers a valuable and fragile cargo: several hundred thousand chicken eggs. In January the race against the clock for the manufacture of the flu vaccine in the Northern Hemisphere begins while that for the Southern Hemisphere is not yet over. In February, the WHO makes its predictions on the dominant strains after months of monitoring the circulation of viruses throughout the world. The vaccine will be manufactured according to the WHO’s recommendations and must be delivered before the start of the influenza season.

The time between the manufacture of a vaccine and its delivery is about two years but for the flu vaccine, everything must move very quickly, with only a few months between the identification of circulating viral strains and the provision of the vaccine in pharmacies. For the Northern Hemisphere, the countdown begins in February, upon receipt of the WHO’s recommendations. The teams then go to work to meet a challenge being constantly renewed with a new race beginning in September for the Southern Hemisphere.
The countdown
From January to May: The millions of eggs have been delivered. Each egg receives a viral strain, then is incubated to allow the virus to multiply. Next, a liquid or serum of virus is harvested, then purified in several stages, filtered and treated to fragment and "kill" the virus. Inactivating a virus so that it is not pathogenic during the injection, in order to induce an immune reaction, is a complex process. The operation is repeated for each of the four viral strains incorporated in the so-called “quadrivalent” vaccine.

The importance of the egg
For vaccine production, the egg remains the most reliable method for rapidly manufacturing large volumes of well tolerated and effective influenza vaccines and responding to global needs. It meets perfectly the flexibility and adaptability required for the vaccine’s manufacture. Nevertheless, for the future, we are exploring new options, including cell production, with the recent acquisition of Protein Sciences, a biotechnology company that has developed the only recombinant protein-based influenza vaccine. This technique will be an alternative that could replace the egg that, for now, remains obligatory.
At each stage, quality control tests are carried out on the four different strains for the purity, sterility and viral concentration that will determine the effectiveness of the future vaccine. "The vaccines are intended for healthy people, requiring the highest standards of quality and safety and increasing the complexity of their production," explains Florence Brunel, Head of Global Programs Execution for Industrial Affairs, Sanofi Pasteur.
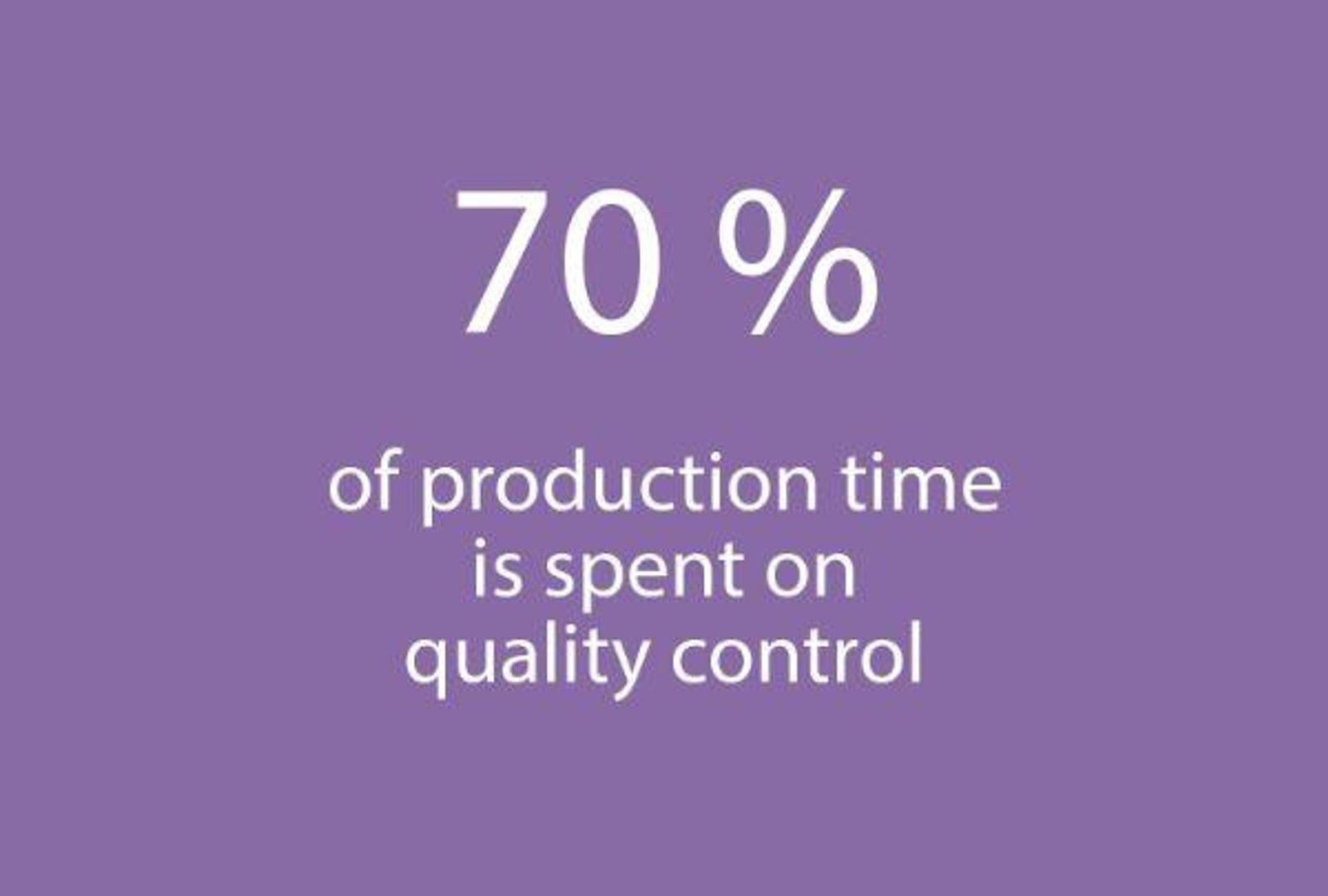
From June to July: The four strains of inactivated virus are collected and combined with each other. New controls, as strict as the previous ones, are made on what is now a vaccine that can be packaged in vials and syringes. Part of the challenge is in the apparent simplicity of a vaccine injection that, in reality, involves an extremely rigorous industrial process. About 70% of the production time is spent on quality tests in addition to those of the health authorities in each country where the vaccine is distributed, so essentially each batch is tested twice.

From July: Production teams, mobilized for weeks, hand over to distribution teams for delivery in the Northern Hemisphere. Whatever the means of transport -- plane, ship or truck -- the cold chain is strictly respected and the vaccines will arrive in time in the pharmacies. As of September and after a new recommendation from the WHO, production will resume for the Southern Hemisphere with the same process. Relentlessly, teams will continue to work to protect people from an illness that kills up to 600,000 people a year, the WHO estimates.

In search of a broadly-protective vaccine
One of the peculiarities of influenza viruses is their amazing ability to mutate. This not only complicates the task of the manufacturer but may reduce the effectiveness of the vaccine when the mutation occurs during the influenza season. Sanofi is actively exploring several of the major influenza vaccination strategies to develop a broadly-protective vaccine designed to address these seasonal changes. Research that is performed internally as well as externally with the University of Georgia, Boston-based biotech company BERG and SK Chemicals, based in South Korea with which we have reached an agreement on the cell culture technology.
"The ultimate goal is to develop a truly universal vaccine; but we expect, as a first step, to replace the current seasonal vaccine, requiring annual administration, with a broadly-protective vaccine," says John Shiver, Senior Vice President, Research and Development at Sanofi Pasteur. "Science, and particularly vaccine R&D, is an iterative process. It's an evolution."
Explore more

On world heart day, it's time for a flu shot
A Look Inside Influenza Vaccines: Today And Tomorrow
Protecting Heart Health: Flu Vaccines and Beyond
Reference
(1) Sanofi Pasteur is Sanofi's Global Business Unit dedicated to vaccines