Harnessing the potential of mRNA to overcome major healthcare challenges
Long before the COVID-19 pandemic catapulted messenger RNA (mRNA) into the spotlight, this essential molecule was already capturing the imagination of scientists looking to solve complex problems in healthcare. After discovering mRNA in 1961, researchers rapidly realized that mRNA could be produced in the lab to express proteins (1980) and envisioned its use in vaccines and other medical applications (1990s).1 By the early 2000s, they began to overcome the roadblocks to introducing this synthetic RNA into the body to prompt the production of proteins involved in various biological activities, from fighting disease to regulating cell functions. Combined with the modifiable nature of mRNA, this capability has opened the door to revolutionizing the way we diagnose, treat, and prevent a wide range of conditions.2
As mRNA research continued to mature over the following two decades2, we at Sanofi recognized a natural application for this technology. As a leading developer of vaccines, we saw that mRNA offered a promising solution to many challenges in vaccinology that we’ve grappled with for more than a century.3 mRNA technology offers a new tool in the vaccine toolbox, where instructions are delivered to certain cells in the body to produce an antigen that acts as a surrogate for any viral or bacterial infection. The antigen then prompts the body to mount an immune response similar to that triggered by natural infection. Since mRNA can code for different proteins, it has the potential to address a wide variety of diseases and pave the way for groundbreaking innovations in biotechnology.2,4
However, we need to address several challenges with existing mRNA technology to fulfill our vision of creating a platform that will become key in routine prevention for multiple diseases. While mRNA has great potential for applications across the field of immunology, it is a very fragile molecule on its own. First, it degrades easily as it is a single strand of nucleic acid. Second, if injected alone into the body, it’s perceived as a foreign entity and attacked by the very immune system it was tasked to reinforce. Furthermore, it needs to go through the impermeable outer layer of our cells: that’s where LNPs come in.
Lipid nanoparticles (LNPs) play a key role as chauffeur and protector of mRNA, helping transport mRNA into target cells while protecting it from destructive enzymes in the body.1,4 LNPs can be best described as small protective bubbles comprised of different types of lipids (fats), which encapsulate mRNA. LNPs have demonstrated their capacity to deliver mRNA to target cells and induce the intended immune response.4
In 2021, we acquired Translate Bio to accelerate the application of multiple LNP technologies and further develop mRNA vaccines for a range of diseases with high unmet needs5.
The fragile nature of mRNA also presents challenges in thermostability.6 Developing mRNA-based vaccines and treatments that remain stable over a range of temperatures would allow for transport over long distances with an increased shelf life and utility for equitable global use. Additionally, we are continuously working on the tolerability of mRNA vaccines, to ensure that any reactions to a vaccine are local, mild, and short-lived, reflecting the current standard of care for today’s non-mRNA vaccines.
The urgent need to protect people against the COVID-19 pandemic catalyzed the rapid advancement of the science in this space, bringing us closer to using mRNA technology for routine immunization and as treatments for certain diseases.
At Sanofi, we are working to unlock the full potential of mRNA. In 2021, we launched our mRNA Center of Excellence (CoE) to accelerate the development and delivery of the next generation of mRNA vaccines 7. Translate Bio brought to the CoE deep knowledge of mRNA science and one of the largest private libraries of LNPs in the world. This, combined with Sanofi’s long-standing experience in vaccine antigen design, immunology and manufacturing and the innovative targeted delivery solution gathered through the acquisition of Tidal Therapeutics, bolstered our efforts to develop a fully owned mRNA platform. Today, we are on track to start at least six mRNA clinical trials by 2025.8 Our goal is to develop vaccines not only for infectious diseases with unmet medical needs, such as chlamydia in adolescents and young adults, and respiratory diseases in older adults, but also to advance treatment for health conditions that still pose a major burden globally, such as acne.8
The Road Ahead for mRNA
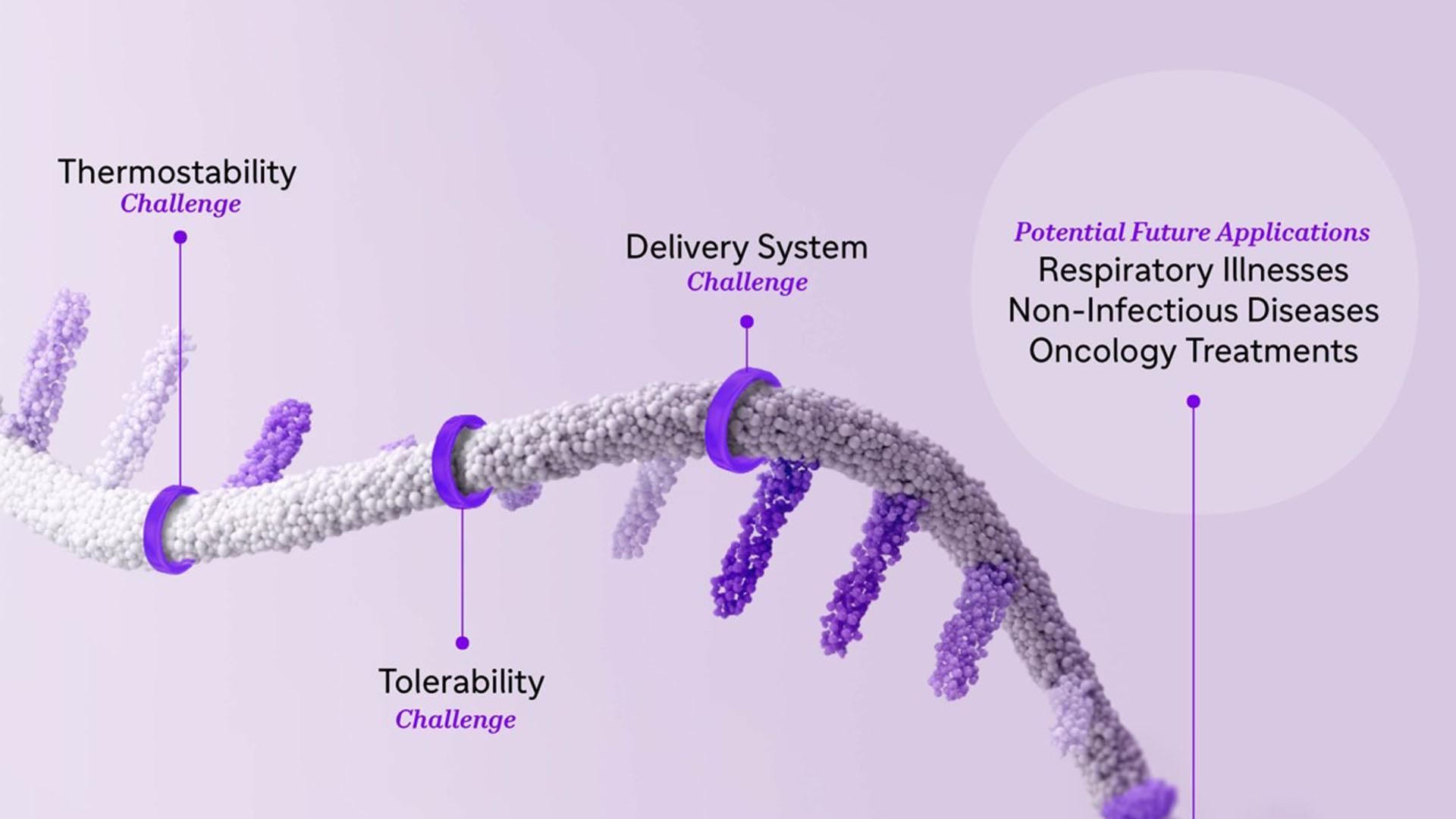
Looking beyond vaccines, we are also leveraging mRNA to develop solutions to therapeutic challenges in oncology, immune-mediated diseases, and rare diseases. For instance, many rare diseases are caused by an inability to produce a certain protein correctly or in sufficient quantities. Here, therapies must be tailored to reach specific cells and repair their ability to make proteins and must also be stable enough to be administered not just once, but routinely, in an outpatient setting. Leveraging the lessons we’ve learned from our vaccine research, as well as our library of proprietary nanoparticles, we have built an mRNA therapeutics platform that will enable our team to develop any type of protein-coding mRNA, as well as the type of LNP needed to deliver it to a specific cell type in the body.
Combining mRNA & LNP technologies opens the opportunity for development in the vaccine area and beyond, like gene therapy and oncology. I believe that it takes creative and passionate scientists working together with strong and visionary leadership to bring mRNA to the next level. This is exactly what you find in the mRNA Center of Excellence.

Régis Gervier
Head of mRNA Center of Excellence at Sanofi
While the field of mRNA therapeutics is still in its infancy, with continued investment and collaboration, it is undoubtedly becoming another valuable platform for pharmaceutical medicines and vaccines development.
References
- Dolgin E. (2021). The tangled history of mRNA vaccines. Nature, 597(7876), 318–324. https://doi.org/10.1038/d41586-021-02483-w
- Kim Y. K. (2020). RNA Therapy: Current Status and Future Potential. Chonnam medical journal, 56(2), 87–93. https://doi.org/10.4068/cmj.2020.56.2.87
- Through time - Sanofi. (n.d.). Www.sanofi.com. https://www.sanofi.com/en/about-us/through-time
- Pardi N., Hogan M. J., Porter F. W., & Weissman D. (2018). mRNA vaccines - a new era in vaccinology. Nature reviews. Drug discovery, 17(4), 261–279. https://doi.org/10.1038/nrd.2017.243
- Sanofi completes acquisition of Translate Bio, accelerating the application of mRNA in new vaccines and therapeutics - Sanofi. (n.d.). Www.sanofi.com. Retrieved February 20, 2023, from https://www.sanofi.com/en/media-room/press-releases/2021/2021-09-14-13-26-25-2296830
- Uddin M. N., & Roni M. A. (2021). Challenges of Storage and Stability of mRNA-Based COVID-19 Vaccines. Vaccines, 9(9), 1033. https://doi.org/10.3390/vaccines9091033
- Sanofi launches dedicated vaccines mRNA Center of Excellence - Sanofi. (n.d.). Www.sanofi.com. https://www.sanofi.com/en/media-room/press-releases/2021/2021-06-29-08-00-40-2254458
- mRNA Technology: Vaccines and Beyond. (n.d.). Www.sanofi.com. https://www.sanofi.com/en/science-and-innovation/research-and-development/technology-platforms/mrna-technology-platform