Fighting Fake Medicines: Our Commitment to Patient and Public Health Protection

Fake medicines and illicit trafficking harm lives. That’s the reason why we have developed a very specific and holistic protection strategy of our products and our patient’s health.
The pharmaceutical crime we are fighting involves illegal activities like medicines’ falsification, or illicitly trafficking medicines, endangering public health and undermining trust in healthcare. Not only are these growing threats on a global scale putting lives at risk, but they are also undermining patient confidence in healthcare systems. This challenge is compounded by the rise of e-commerce, which has opened up new avenues for counterfeiters and traffickers to reach unsuspecting patients.
What Is a Falsified Medicine?
"Falsified medicines are medical products that deliberately or fraudulently misrepresent their identity, composition or source."
WHO Definition
Pharmaceutical Crime Affects the Whole World
Geographic Distribution of Incidents* (2022)
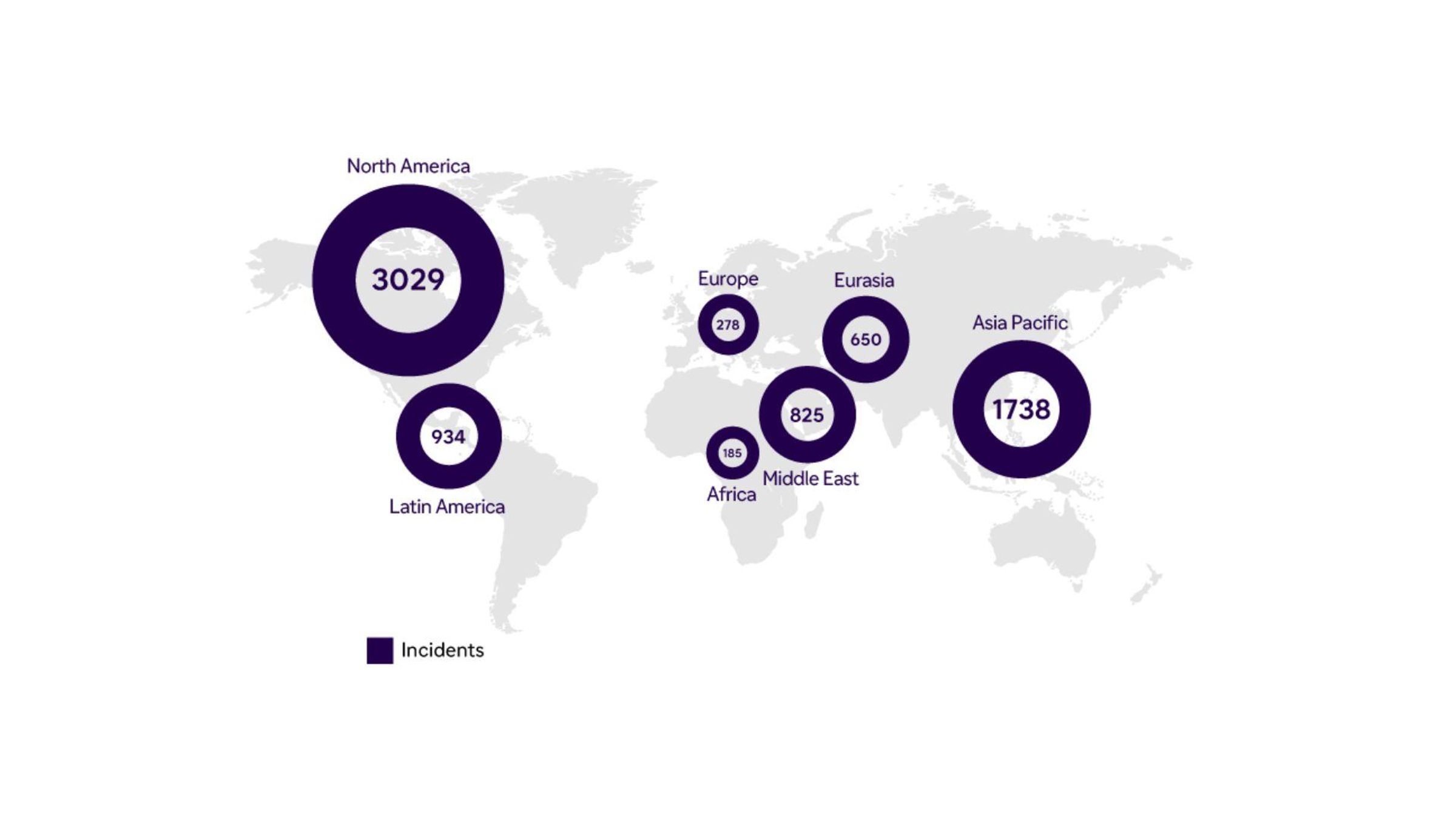
*Definition of an incident (falsification + illicit diversion): tracks news and unique activity; verified information; single case can involve multiple incidents; single incident may involve multiple detections.
Key Figures
$4.4bn
A United Front Against Pharmaceutical Crime
We combat global pharmaceuticals crime with a global-to-local approach.
Tackling this challenge is not just a responsibility; it’s a commitment that drives us forward. For decades, we’ve been at the forefront of combating falsified medicines, standing as a trusted partner to protect patients and communities worldwide.
To do so, we innovate at every step, using secure packaging and cutting-edge digital tools to fight falsification. Our digital authentication systems help patients and healthcare providers verify medicines' authenticity in seconds, while advanced monitoring software scans the web for illicit activities, enabling real-time interventions.
Collaboration drives our impact. With academic alliances, like our work with the Paris Saclay Foundation, we push boundaries to go beyond criminal tactics, by combining our strenghts with those of Paris-Saclay and enhance our efficiency in data analysis and innovative technical solutions for authentication and traceability.
The quality of our product is safeguarded only when it is genuine and legitimately distributed. That's why the fight against falsified medicines and illicit trafficking is essential to protecting our products and our patients.

Michel Sebah
Global Product and Patient Protection Head - Corporate Security
Beyond addressing immediate threats, we prioritize prevention. By closing supply chain vulnerabilities, raising public awareness, and embracing innovation, we aim for lasting solutions that safeguard health for everyone.
At Sanofi, we recognize that addressing these challenges requires urgent and coordinated action. Our strategy combines state-of-the-art technology, collaborative partnerships, and sustainable practices to provide a comprehensive response to these critical issues. It is grounded in two key axes:
- Protecting our products through innovative safeguards and supply chain protection.
- Protecting patients and public health in an increasingly digital world by disrupting counterfeit sales online and raising awareness.
Securing the Integrity, Authenticity and Identification of Our Products
Industrial Product Protection
Three types of complementary procedures are used to guarantee global protection of our assets.
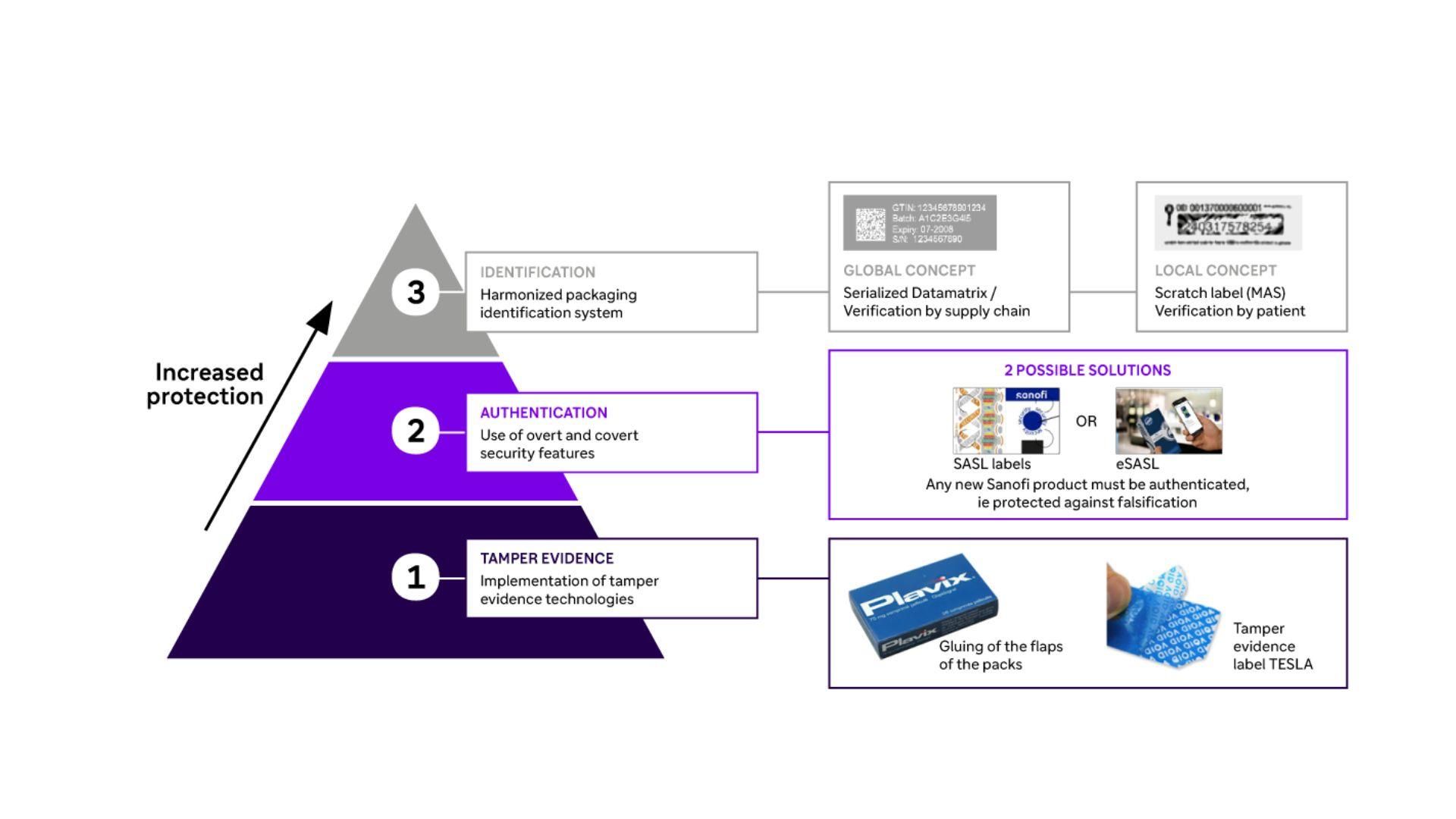
Innovative security measures: From tamper-proof seals to unique serialization codes
Protecting Patients in a Digital World
Expanding online healthcare increases the risk of fake meds
- Advanced online monitoring: our sophisticated tools detect illicit offers in real time, enabling us to intervene quickly and decisively: we have developed a robust integration solution that leverages advanced technologies to ensure safe access to genuine medicines.
- Collaborations with online platforms: Working with leading e-commerce and social media platforms, we ensure illicit offers are removed, safeguarding patients wishing to buy medicines online across the globe.
- Public awareness campaigns: By educating communities about the dangers of unauthorized online pharmacies, we empower patients to make safer choices.
A Shared Mission for Public Health
At Sanofi, protecting Public Health is not just a priority—it is a commitment that drives every aspect of our work. We participate to a safe access to genuine medicines legitimately distributed by combating falsified medicines. But it requires collaboration at all levels: governments, regulators, NGOs, and patients. It also requires the power of AI: that is why we use AI-driven data integration to detect threats in real-time, ensuring patient safety through faster, proactive, and effective decision-making. Together, we can create a world where safe, high-quality medicines are accessible to all, and where trafficking has no place.