Targeting Neuroinflammation in Multiple Sclerosis
Multiple Sclerosis (MS) is a chronic, disabling disease affecting more than 2.3 million people worldwide. We asked Tim Turner, Global Project Head for Neurology at Sanofi, about the big challenges in MS research today.
Inflammation has come into focus across many diseases, and neurology is no exception. Our R&D teams are focused on developing drugs that target neuroinflammation: the overactive immune response that drives inflammation and disease progression behind the blood–brain barrier.
Just 30 years ago, there were no approved therapies for MS. Today, there are over a dozen disease-modifying therapies available to patients that have been proven to reduce the frequency of relapses, and even the accumulation of disability. This reflects major advances in our understanding of immune responses that drive the disease. The big challenge in MS research now centers on controlling the disease process directly within the central nervous system (CNS), as well as outside it, to halt its progression.

Timothy J. Turner, Global Program Head for Neurology at Sanofi
How MS affects the brain
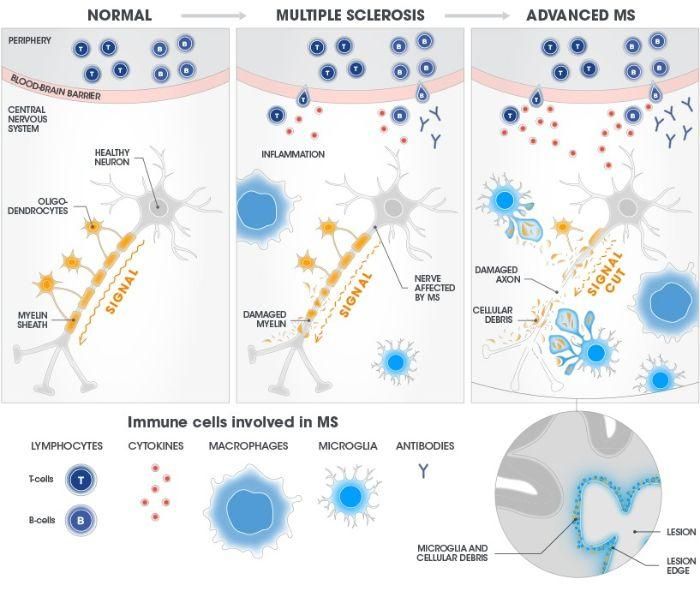
In MS, the immune system attacks healthy tissue and causes damage to the brain and spinal cord for reasons we don’t understand. The defining feature of this autoimmune attack is the damage it wreaks on the myelin sheath inside the CNS. This protective covering surrounds the long, cable-like axons that conduct electrical signals between neurons, which carry information between the brain and other parts of the body in a massive network.
Neuroinflammation breaks the myelin sheath apart in a process called demyelination, and this can ultimately damage the axon, cutting the connection between neurons. These degenerative processes can start early in MS, before a patient—or someone in their life—spots any signs of the disease, such as lack of coordination or difficulty walking.

Bringing New Hope to People Living with Multiple Sclerosis
Dov, who is living with MS in Israel, inspires scientists to advance care by taking on the big challenges in research
How neuroinflammation starts
There are always immune cells working inside the brain: resident immune cells carrying out routine maintenance, and immune cells from the periphery that cross the blood-brain barrier to carry out “immune surveillance”. In MS, both types can become aggressive and malfunction:
- T-cells, antibody-producing B-cells, and other immune cells that cross the blood–brain barrier can attack the brain and spinal cord.
- Within the CNS and outside of it, B-cells release substances called cytokines that trigger inflammation.
Microglia regularly patrol the brain to maintain a "clean room" environment. In MS, they go into overdrive and cause damage to the myelin sheath. This creates debris, and microglia respond. Lesions composed of microglia, myelin debris, and other cells accumulate at the lesion's edge, where they can be detected by advanced MRI techniques.
Chronic lesions
The resulting damage in the brain can be detected using advanced MRI techniques: they appear as lesions, with immune cells and myelin debris clustered along the edges. At that point, one of three things can happen to the axons:
- they could become re-myelinated;
- they could become inactive without being remyelinated; or
- they might continue to degenerate.
Some lesions represent sites of chronic, 'smoldering' inflammation in the brain. These smoldering lesions have been associated with brain volume loss, disability, and disease progression.
Compounding the damage
When cells in the CNS are destroyed, the resulting debris attracts the attention of innate immune cells like microglia, which normally have a "clean up" role inside the CNS. Unfortunately, this can cause further damage.
Microglia reside in the brain and spinal cord, and actively maintain the health of the CNS. Among many beneficial functions, they patrol for signs of cellular damage or pathogens that trigger an innate response. In MS, what starts out as a protective function becomes a destructive one: the debris from demyelination causes microglia to go into overdrive and destroy normal tissue. This neuroinflammatory process may contribute to further myelin destruction. It creates a hostile environment and prevents repair processes that would normally foster re-myelination.
To limit or repair the damage, it is crucial that we find ways to control it. Because microglia reside in the CNS, any potential treatment would need to cross the blood–brain barrier to affect them.
Crossing the blood–brain barrier
The blood–brain barrier keeps tight control over everything that enters or leaves the CNS. It's one reason most MS treatments, for example antibody therapies, are designed to target cells outside the CNS: blocking unwanted T-cells and B-cells from entering the brain can actually limit some degenerative activity inside the brain.
Developing a treatment that could cross the blood–brain barrier, get into the CNS and act directly on immune cells is an important frontier in research at Sanofi. As we understand more about MS, we can focus on specific targets within the brain that haven't been accessible before.
Targeting BTK
For any drug to enter the CNS, it must evade the body's sentinel system, which blocks unwanted substances from entering the brain. Sanofi scientists are investigating ways to target an enzyme called Bruton's tyrosine kinase (BTK).
BTK is critical to the activation of many immune cells that drive MS, such as B cells and microglia, and has gained attention for the role it plays in immune activities on both sides of the blood–brain barrier. That's why we believe that a treatment that could inhibit BTK within the brain may be able to calm the activity of microglia and other immune cells, shifting them back into a more normal state.
Our neurology teams are investigating a BTK inhibitor designed to cross the blood-brain barrier in humans. Our hypothesis is that blocking BTK in the CNS will modulate microglia and B-cells inside the brain, and that it might be possible to modulate B-cells and microglia in the CNS. That could reduce the impact of the disease on people living with MS. That would mean a lot for the patient community.
Timothy J. Turner
Global Project Head for BTKi at Sanofi
Looking ahead
The scientific community has known that MS is a demyelinating disease of the brain since the 1870s, but only recently has it become possible to target over-active immune cells in the brain. New approaches to chemistry and novel methods to "shuttle" proteins across the blood–brain barrier have reset expectations for what is possible in neurology research. Our teams are leveraging these new technologies to pursue a new generation of therapies that would have been unattainable just a decade ago. And that is a really exciting place to be.
By Timothy J. Turner, Global Program Head for Neurology at Sanofi
Explore more

Supporting Children whose Parents have Multiple Sclerosis
Pioneering Technology Platforms
Neurology R&D
References
- Franklin, R., ffrench-Constant, C (2008) Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci 9:839–855; doi: 10.1038/nrn2480
- Li R, Patterson KR, Bar-Or A (2018) Reassessing B cell contributions in multiple sclerosis. Nat Immunol 19:696–707; doi: 10.1038/s41590-018-0135-x
- Liddelow SA, et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481-487; doi: 10.1038/nature21029
- Ludwin SK, Rao VTs, Moore CS, Antel JP (2016) Astrocytes in multiple sclerosis. Mult Scler 22:1114-1124; doi: 10.1177/1352458516643396
- Reich DS, Lucchinetti CF, Calabresi PA (2018) Multiple Sclerosis. N Engl J Med 378:169-180; doi: 10.1056/NEJMra1401483
- Reich DS, et al. (2021) Lancet Neurol 20:729-238; doi: 10.1016/s1474-4422(21)00237-4
- Trapp BD, et al. (1998) Axonal transection in the lesions of multiple sclerosis. N Engl J Med 338:278-285; doi: 10.1056/NEJM199801293380502
- Weber ANR, et al. (2017) Bruton’s tyrosine kinase: an emerging key player in innate immunity. Front Immunol 8:1454; doi: 10.3389/fimmu.2017.01454
- Werneburg S, et al. (2020 Immunity 52:167-82.e7; doi: 10.1016/j.immuni.2019.12.004
- Wiendl H, Gross CC (2013) Nat Rev Neurol. 9:394-404; doi: 10.1038/nrneurol.2013.95