In the Spotlight
Our Science
January 26, 2026
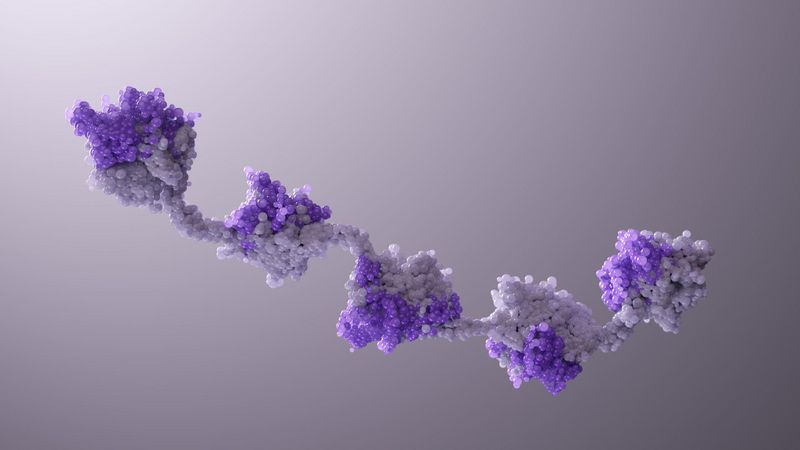
Two’s Company. Three’s a Crowd. Five Nanobody® Subunits? That Could Be a New Medicine.

Our Science
January 13, 2026
AI Across the R&D Value Chain: Manufacturing - Digital Labs and Self-Sharpening Tools

Our Science
January 6, 2026
Our Human-Centric Approach to Partnership Amidst an Evolving Biotech Landscape
Watch Our Latest Videos

AI in Manufacturing & Supply

How is AI Speeding up Drug Discovery?

Is AI a Superpower?

The Sanofi Connection
All Stories
Year
Topic
Your Health
March 9, 2026
More Than Multiple Myeloma; Understanding the Unseen Burdens
Our Science
February 24, 2026
AI Across the R&D Value Chain: Portfolio Decision-Making
Sustainability
February 19, 2026
Strengthening Djibouti’s Fight Against Non Communicable Diseases
Our Science
January 26, 2026
Two’s Company. Three’s a Crowd. Five Nanobody® Subunits? That Could Be a New Medicine.
Our Science
January 13, 2026
AI Across the R&D Value Chain: Manufacturing - Digital Labs and Self-Sharpening Tools
Your Health
January 6, 2026
Defining a Clearer Path Forward in CIDP: Why Language Matters
Our Science
January 6, 2026
Our Human-Centric Approach to Partnership Amidst an Evolving Biotech Landscape
Your Health
December 17, 2025
The Cause of COPD You Don't Know: Alpha-1 Antitrypsin Deficiency
Sustainability
November 24, 2025
