REGAINing Control of Asthma
A precision immunology collaboration captures the real-world health journeys of asthma patients, combining Internet of Things (IoT), clinical, and genomic data to see the disease with new eyes.
Asthma affects 300 million people worldwide and is the most common chronic condition among children. Asthma attacks, while treatable, can be sudden and severe, and if left unchecked they can be fatal.
“Every parent who has a child with asthma is worried about the attack that leads to the emergency room or even the ICU,” said Frank Nestle, Sanofi's Head of Research and Chief Scientific Officer. “But what if we can help create a world where no one has to suffer from an asthma attack anymore?”
Many patients suffer from asthma driven by type 2 inflammation, an immune response that evolved as a defense against parasites but can go into overdrive and trigger allergic conditions. Type 2 inflammation research is critical for discovering new ways to tame asthma and other allergic conditions. But it is not the only driver of the disease.
Sanofi researchers are taking a precision immunology approach to understanding asthma, with the goal of taking asthma diagnosis and treatment to new levels. One example of this work is the REGAIN study: a project led by Sanofi and health intelligence company Sema4, leveraging asthma management technology from Cohero Health.
The REGAIN study captures the real-world health journeys of hundreds of asthma patients, from all walks of life, at Mount Sinai Health System in New York City. By combining real-world health data with clinical and molecular information, the study aims to light a path to new medicines and empower patients to head off asthma attacks before they happen.
Capturing the patient journey
The 1,200 people participating in REGAIN include around 800 people living with asthma and 400 healthy people who act as controls (see box). Patients in the study are using Bluetooth-enabled inhalers and peak-flow spirometers connected to a mobile app, which measure and record both patient medication use and lung function, in their daily lives. The solution, developed by Cohero Health, is a secure app on the patient's ordinary mobile phone collecting environmental information including temperature, humidity, pollen count, and pollution. It is also connected to a physician’s office to provide visibility to those patients who may need follow-up.

Patients in the REGAIN study are using Bluetooth-enabled inhalers and peak-flow spirometers connected to a secure mobile app on an ordinary mobile phone, which collects environmental information and is connected to a physician’s office
In this virtual setup, patient participants can share accurate, highly detailed information about their experience with asthma directly, without having to remember all the details later.
“As a community, we are studying this disease in much more depth and dimension than ever,” said Paul Bryce, Senior Director of Type 2 Inflammation and Fibrosis, who is leading the study for Sanofi. “The scale of this asthma study, encompassing real-world data and molecular profiling, is unprecedented. Mobile and molecular technologies are helping us gain insights into asthma in all its forms, which is critical to finding new drug targets–and that is a big part of what we hope to achieve."
Real-world, clinical, and genomic data
Over 18 months, REGAIN's remote measurement devices will generate valuable data about how and when patients use their asthma medication, under what conditions. Along with clinical and molecular data, they will also provide information about how a patient's body responds to treatments. Researchers will combine all real-world, clinical, and molecular data, using bioinformatics and machine learning to discover meaningful patterns.
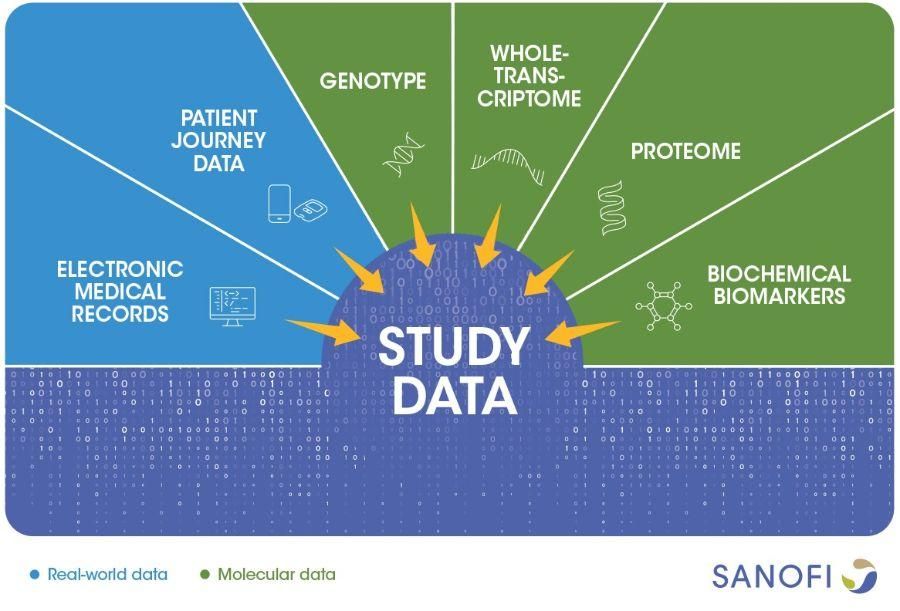
This will give a detailed, nuanced picture of a how asthma unfolds in different groups of people, which could help physicians match patients to treatments more accurately. It could also point to promising drug targets, the first step in developing new treatments.
"Asthma is an umbrella term for many different disorders with the same symptoms," explained Emanuele de Rinaldis, Senior Director and Head of Precision Immunology at Sanofi. "Currently, research and treatments focus on two clinical endpoints: the amount of air patients can blow out of their lungs in one second, and the number of asthma flare-ups they experience in a year. REGAIN is measuring many more dimensions of the disease–everything from DNA sequencing and gene expression to how patients are feeling throughout the day–so that we can find common factors in groups of people. And that will point the way to targeted treatment and prevention."
Rachel Sha, Vice President and Head of Digital Strategy and Governance, added, “We want to unlock deeper insights into asthma so that we can develop better, more targeted solutions and therapies in the future. To do that, we have come together with world-class experts in respiratory disease, computational disease modeling, digital health, and biology. Collaborating with Sema4, and the Principal Investigator at Mount Sinai, is essential to the success of this project.”
Anticipating attacks
In addition to discovering new drug targets in REGAIN, Sanofi scientists and their colleagues hope to take one step back and pinpoint the onset of an asthma attack.
“Part of REGAIN is about identifying common factors–biomarkers–that we could use to help predict an asthma attack, rather than minimizing its impact after the fact,” said Nestle. “We want to understand the journeys of asthma patients in the real world because that’s how we’re going to truly change the treatment paradigm for this disease.”
REGAIN patient journey data
- Environmental factors (e.g. weather, pollen count)
- Digital biomarkers (e.g. sleep, activity)
- When medications are taken
- Night awakenings
- Timing and severity of asthma attack
- Patient-recorded outcomes, symptoms, and triggers
REGAIN molecular data
- Genotyping
- Whole-transcriptome sequencing (nasal brush and whole-blood)
REGAIN clinical data
- Electronic Health Records (consistent measures)
REGAIN participants
- People with "type 2 driven" asthma managed well by biologics
- People with type 2 driven asthma managed well by other treatments
- People with type 2 driven asthma not managed well
- People with "non-type 2 driven" asthma
- Healthy people who act as controls
Explore more
The Next Breath: Advancing Knowledge to Transform Asthma Care

Asthma and Whooping Cough don’t Mix
Immunology and Inflammation R&D
References
- Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–59.
- Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2016. Geneva, World Health Organization; 2018.
- Global Health Estimates 2016: Disease burden by Cause, Age, Sex, by Country and by Region, 2000-2016. Geneva, World Health Organization; 2018.