Vaccines and Monoclonal Antibodies in Immunization
Monoclonal antibodies–human immune proteins–can be designed to provide rapid protection against some infectious diseases.1,2,3 As families throughout the world seek to defend themselves against high-profile diseases such as COVID-19, influenza, and respiratory syncytial virus (RSV),4,5 scientists are exploring how they could help expand the immunization toolkit.
Vaccines help the body make its own antibodies
Antibodies are one of our most important defenses against infectious diseases. They are built to recognize viruses, bacteria, and abnormal cells, and to flag them for attack by the immune system. These Y-shaped proteins are produced by white blood cells called B cells. An antibody must have very specific properties to lock on to a particular virus, for example, and neutralize it successfully.
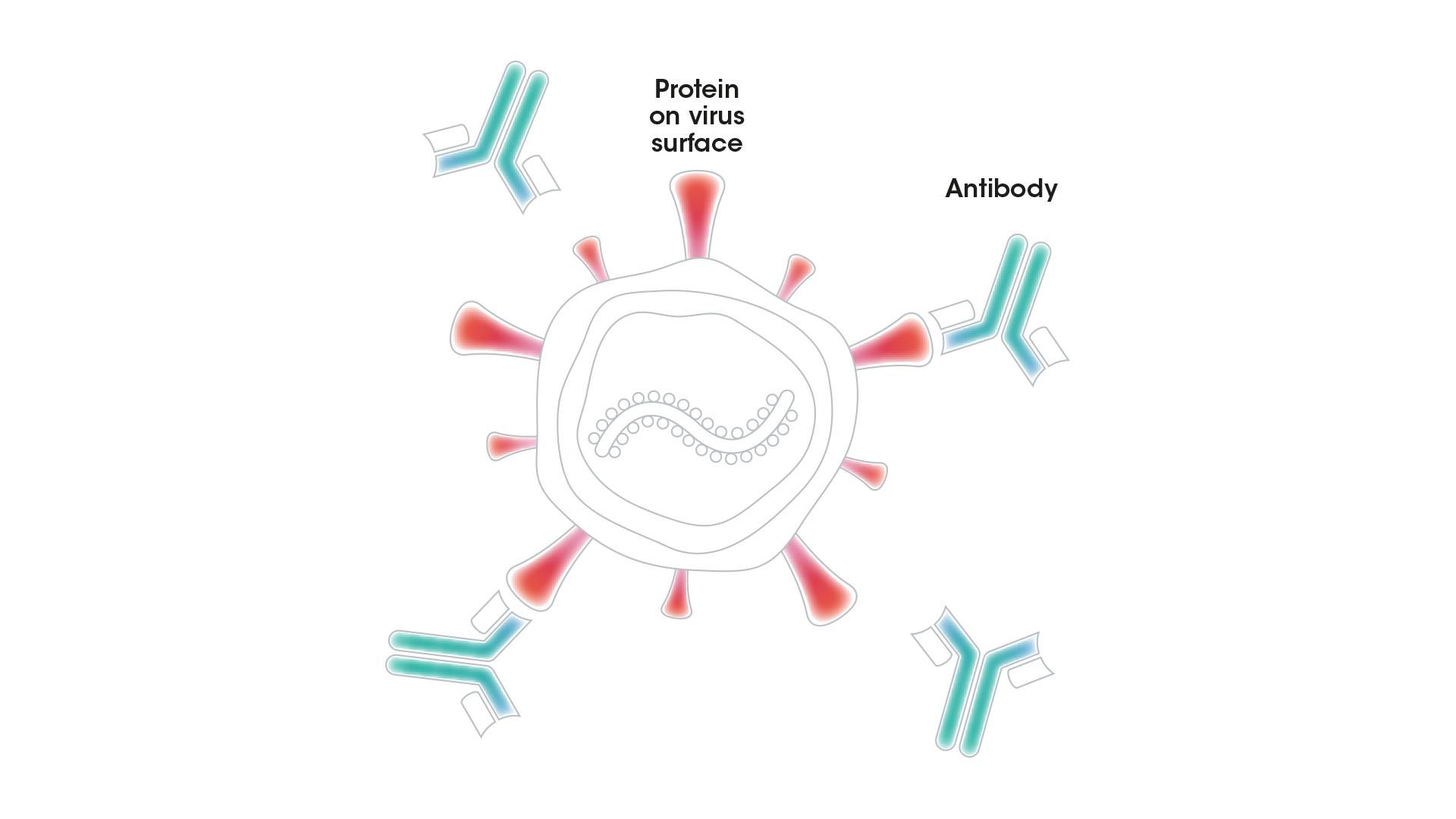
An antibody with the right properties can bind to a protein on the surface of a virus and block it from entering a person's cells or destroy it directly
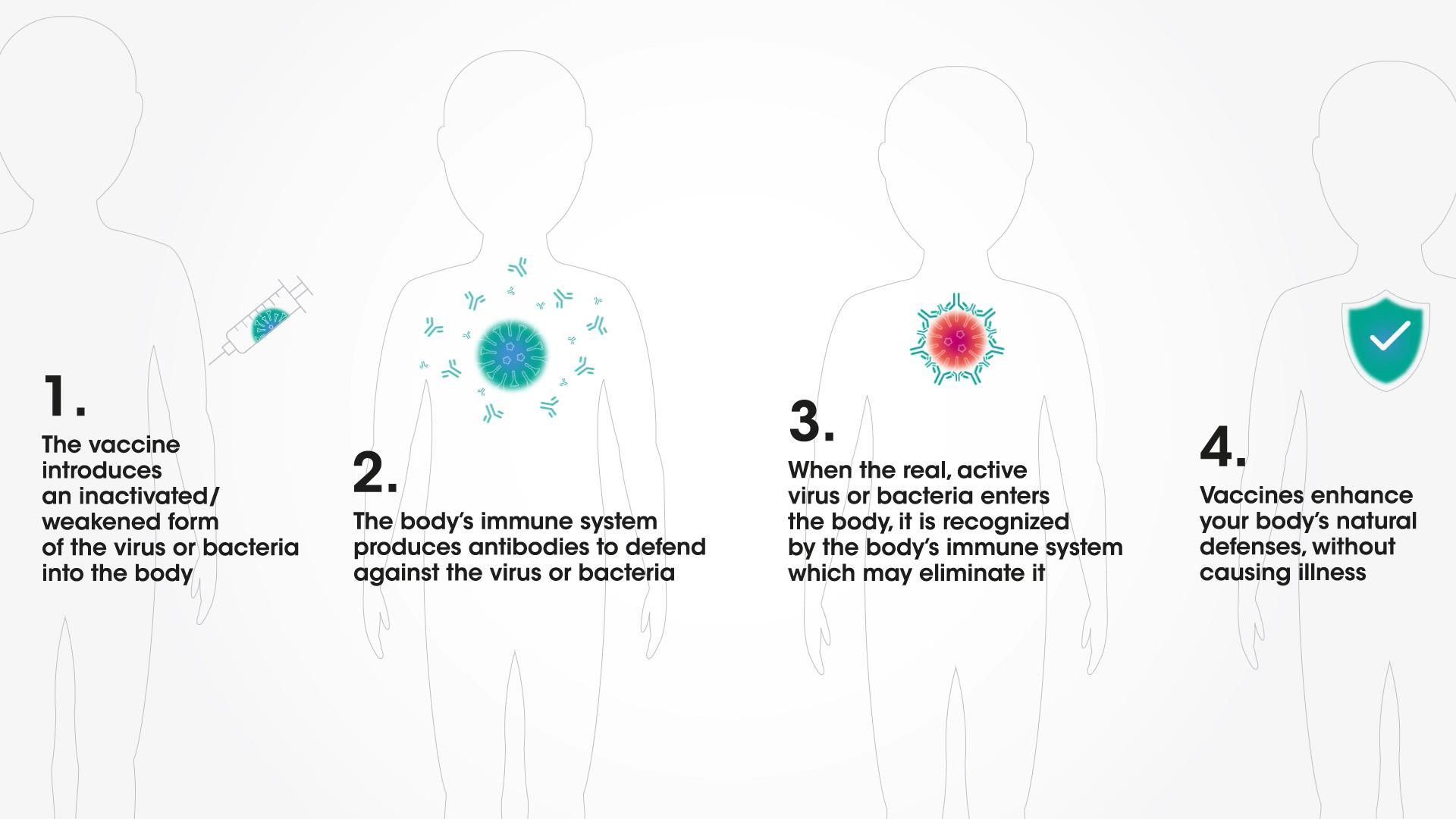
From infancy, the body can make antibodies to fight infection6–including with the help of vaccines. Following vaccination, it is only a matter of weeks until the body learns how to mount its own immune response against a particular viral infection. Vaccines are designed to train the body to remember this defensive response for many years.7 That's why they are a cornerstone of public health.
How vaccines work
Monoclonal antibodies could help provide rapid protection
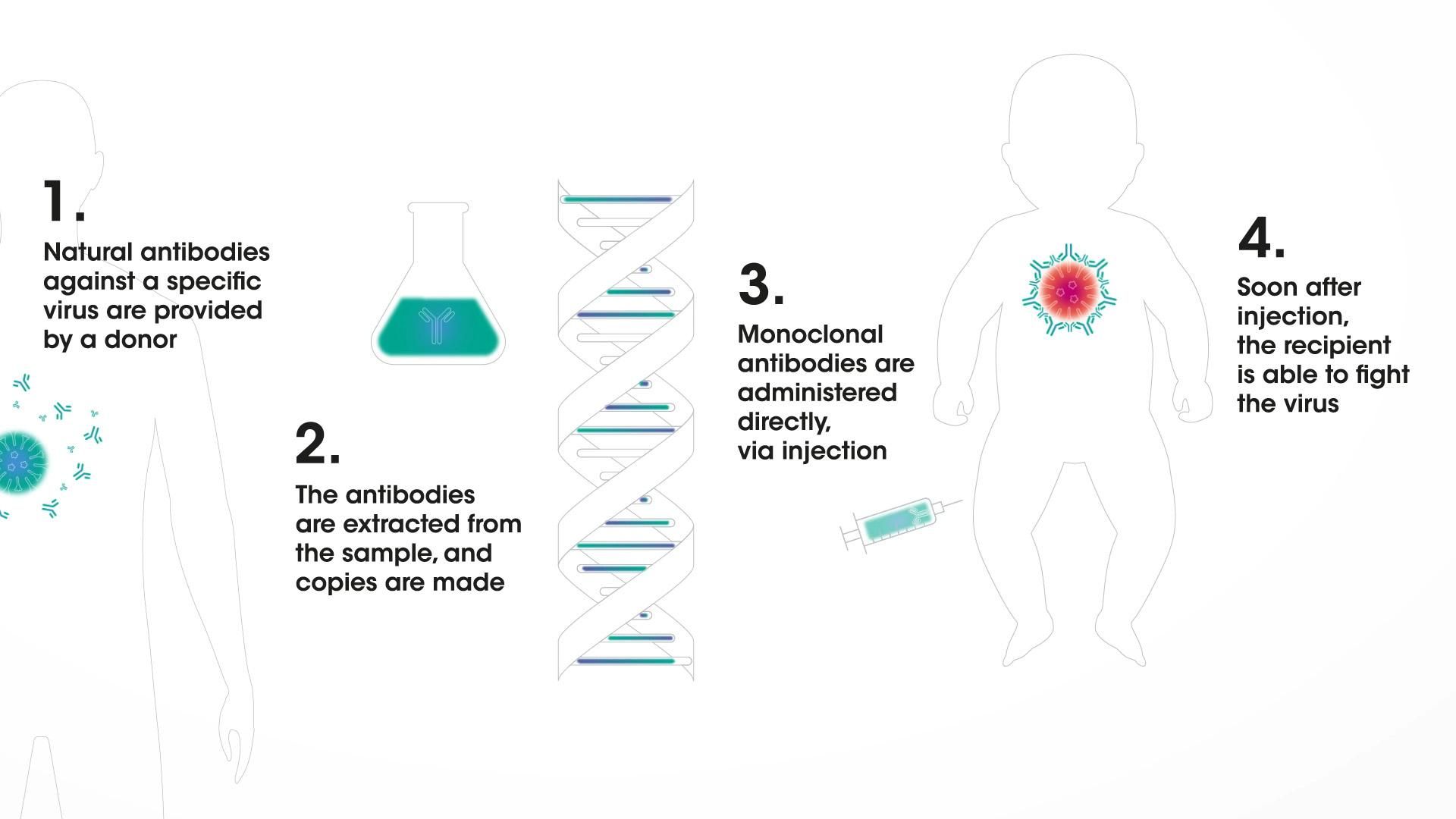
In a broad immunization program, vaccines could one day be augmented by monoclonal antibodies that could offer direct protection. Monoclonal antibodies are human immune-system proteins that can be designed to do a very specific job: to be "ready to go" and provide protection soon after administration against a disease.8,9,10 That means they could serve as a line of defense for people who are susceptible to infection, such as infants.
Monoclonal antibodies in immunization
The goal: Bridging gaps in infant immunity
The direct protection potentially offered by monoclonal antibodies could bridge important gaps in immunity, for example when infants are too young to be protected by a vaccine, or when no vaccine is available. Monoclonal antibodies could also help protect infants when infectious diseases spread during their first year of life.11,12,13
Scientists are working to extend the amount of time a monoclonal antibody could circulate in the body – potentially for as long as several months. That development is empowering researchers to pursue different ways to protect infants during their most vulnerable time of life.
Explore more
mRNA Technology: Vaccines and Beyond

The Upshot: What is RSV?

Vaccines: Innovation for Disease Prevention and Control
References
- Rodriguez-Fernandez R, Mejias A, Ramilo O (2021) Ped Infect Dis J 40:S35-S39. doi: 10.1097/inf.0000000000003121
- Group TPIW, Team M-NPIS (2016) N Engl J Med, 375:1448–1456; doi: 10.1056/nejmoa1604330
- Domachowske JB, et al. (2018) Pediatr Infect Dis J. 37:886-892; doi: 10.1097/INF.0000000000001916
- Fox, M (2021) CNN news article, accessed 11 February 2022
- Rodriguez A (2021) USA Today news article, accessed 11 February 2022
- Tsafaras GP, Ntontsi P, Xanthou G (2020) Front Pediatr 8:5; doi: 10.3389/fped.2020.00005
- Centers for Disease Control and Prevention (2018) Understanding How Vaccines Work, accessed 11 February 2022
- Malik B, Ghatol A. Understanding How Monoclonal Antibodies Work. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021 Jan. Accessed 11 February 2022
- Bayer V (2019) Sem Onc Nursing 35:150927; doi: 10.1016/j.soncn.2019.08.006
- Marston HD, Paules CI, Fauci AD (2018) N Engl J Med 378:1469-1472; doi: 10.1056/NEJMp1802256
- Rodriguez-Fernandez R, Mejias A, Ramilo O (2021) Ped Infect Dis J 40:S35-S39. doi: 10.1097/inf.0000000000003121
- Prevail II Writing Group (2016) N Engl J Med 375:1448–1456; doi: 10.1056/nejmoa1604330
- Caskey M, Klein F, Lorenzi JC, et al. (2015) Nature 522:487–491; doi: 10.1038/nature14411
Further reading
- European Vaccination Portal (2021) How Vaccines Work, accessed 11 February 2022
- Law M and Hangartner L (2008) Curr Opin Immunol 20:486-492; DOI: 10.1016/j.coi.2008.06.005
- National Institutes of Health News (2018) Monoclonal Antibodies Crucial to Fighting Emerging Infectious Diseases, Say NIAID Officials, accessed 11 February 2022
- U.S. Centers for Disease Control (2021) The Immune System—the Body’s Defense Against Infection, accessed 11 February 2022
- U.S. Centers for Disease Control (2017) Immunity Types, accessed 11 February 2022