Decade Commitment to Innovation in Hemophilia Continues

A hemophilia diagnosis can often bring uncertainty to everyday life. While there has been significant progress made in the treatment landscape, unmet needs remain for people living with the disease across the world.
There are few rare blood disorders that have seen treatment progress like hemophilia. This year marks a decade since the introduction of a new generation of therapies offering reliable bleed protection with more convenient dosing for people living with hemophilia. Today, more than one million people around the world are estimated to be living with either hemophilia A (factor VIII deficiency) or hemophilia B (factor IX deficiency).1,2 But the broader history of hemophilia treatment has also seen significant advancements.
Few people know this better than Phil, 68, who upon hearing his diagnosis of moderate hemophilia in 1970, felt a sense of both shock and relief.
I had mixed feelings about my diagnosis. On the one hand, I was grateful to finally have a name for what I was experiencing. I felt validated and less paranoid. But I also had to grapple with what my doctors told me: that I would be really lucky to live past 20, due to the limited treatment options we had back then. Words fail to describe how heavy that feeling was.

Phil
living with hemophilia
The feeling Phil describes was often the harsh reality for people living with moderate to severe hemophilia. This condition prevents the blood from clotting properly and causes uncontrolled bleeding, leading to joint damage and chronic pain.2 Without access to treatment and if left uncontrolled, it may become life-threatening.2 In the 1960s, life expectancy for people living with hemophilia increased to 24 years, and the first clotting factor concentrate was developed.3 This would remain the standard of care until the 1990s when recombinant products were introduced, which eliminated dependency on human-blood-derived therapies and facilitated the introduction of regular prophylaxis.3 But there was still a significant treatment burden of frequent infusions.
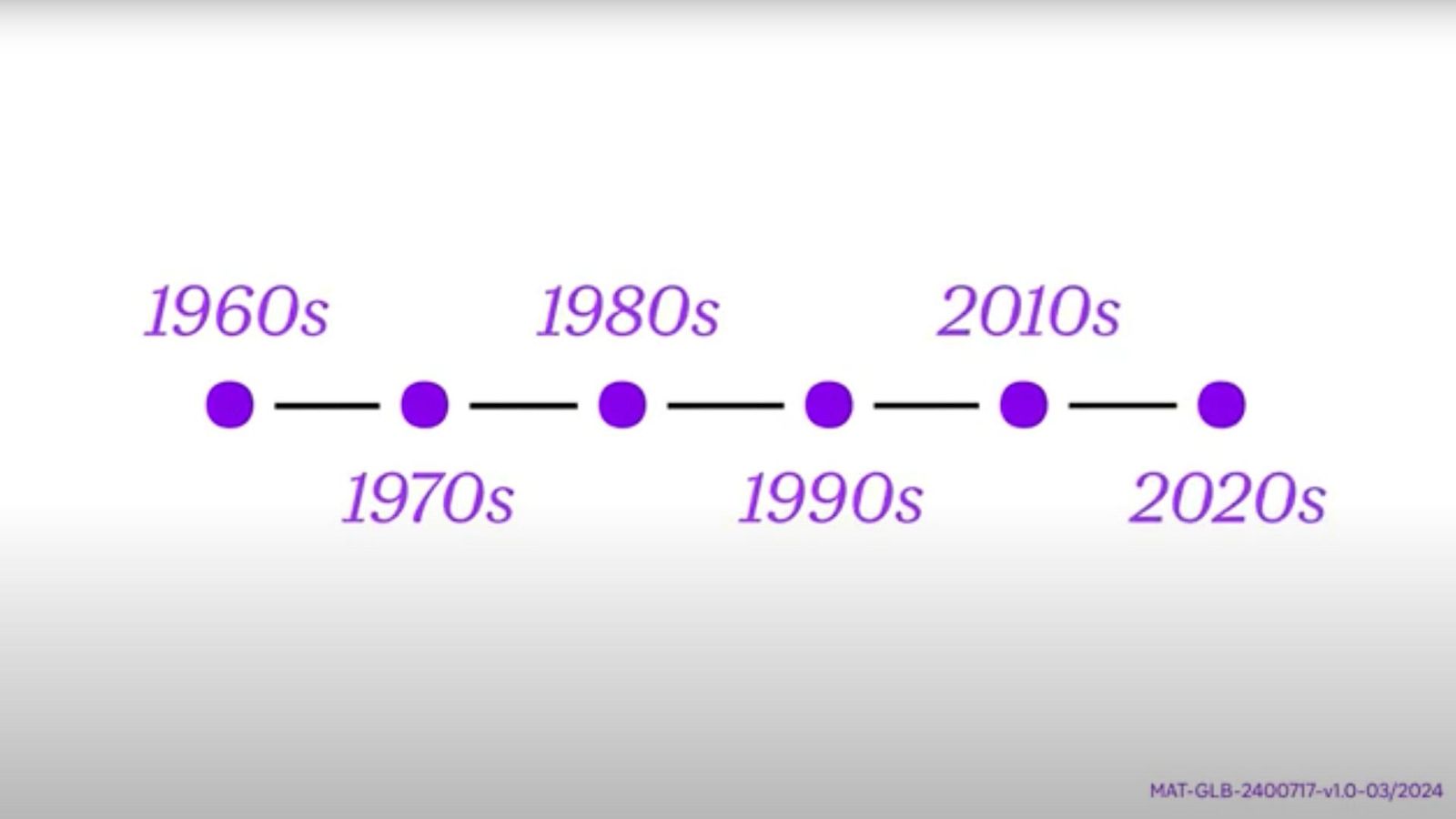
Ten years ago, there was a dramatic transformation in the treatment landscape. In 2014, with our partner Sobi*, we developed first-in-class extended half-life factor therapies (EHL). EHL factor treatments work by prolonging the amount of time that factor (proteins that control clotting) is present in the blood, offering more convenient and flexible dosing options.4
"Living with hemophilia has never been easy, but medical advancements have greatly evolved in my lifetime. We’ve come a long way, and I am ecstatic that there is more awareness and understanding of hemophilia than there was when I was a kid."
-Phil, living with hemophilia
At Sanofi, we are building on the foundation set in 2014 by continually challenging the status quo to redefine what is possible for people living with hemophilia. Over the last decade, we have focused our research and development on potential new treatment options that provide consistent bleed protection while continuing to reduce the burden of treatment.
The advancement of hemophilia treatment has made life easier for people with this disorder, and we’re actually now seeing that the life expectancy for people who live with hemophilia is approaching that of people who don’t have it. We’ve made incredible strides in treating hemophilia, but we have a long way to go.

Dr. Dave Clark
Chairman of the Coalition for Hemophilia B
Our Commitment
While the treatment landscape has continued to evolve, sustainable access to care remains a challenge for many people living with this disease. In fact, more than 75% of the global hemophilia community have limited or no access to diagnosis or treatment, creating a public health challenge that must be addressed.5
In 2014, the same year EHL factor therapies were introduced, we responded to an industry call to action from the World Federation of Hemophilia (WFH), together with Sobi*, to help address the treatment gap for people living with hemophilia in emerging countries.
Together, the companies committed to donating more than one billion IUs of factor therapy to the WFH Humanitarian Aid Program over ten years, resulting in the single largest donation of hemophilia factor therapy. Through this commitment, the WFH has been able to provide a sustainable supply of therapy not only for life-saving surgeries and acute bleeds for people with hemophilia but also prophylaxis in people, mostly children, around the world who do not have access to consistent treatment.
The Next Generation of Innovation
To shed more light on the impact of hemophilia and the remaining unmet needs among the community, we recently partnered with The Harris Poll to conduct a global survey of patients, caregivers, and providers about their experiences with hemophilia. By better understanding the evolving needs of the hemophilia community, we can continue innovating to help address unmet needs and optimize hemophilia care.
Our hope is that in another decade, we can once again look back and celebrate how much further we have come in advancing care and improving outcomes.
With our continuous commitment to the hemophilia community, together we can get there.
Explore more

June 22, 2023
Following the Science to Improve Care in Rare Blood Disorders
References
- Iorio A, Stonebraker JS, Chambost H, et al. Establishing the Prevalence and Prevalence at Birth of Hemophilia in Males: A Meta-analytic Approach Using National Registries. Ann Intern Med. 2019;171(8):540-546.
- Center for Disease Control (CDC). What is Hemophilia? Accessed February 21, 2024.
- Hemophilia Federation of America (HFA). History of Bleeding Disorders. Accessed February 21, 2024.
- Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313-2323.
- Sanofi’s Humanitarian Programs Help Patients Worldwide. Sanofi.com. Accessed February 21, 2024.
*Sobi is a specialized international biopharmaceutical company transforming the lives of people with rare and debilitating diseases. Sobi and Sanofi collaborate on the development and commercialization of three hemophilia therapies. Sobi has final development and commercialization rights in the Sobi territory (essentially Europe, North Africa, Russia and most Middle Eastern markets). Sanofi has final development and commercialization rights in North America and all other regions in the world excluding the Sobi territory.